DuPont Announces Tyvek Manufacturing Change
Tyvek Manufacturing Process Changing
ARLINGTON, Va., Aug. 24 -- The Association for the Advancement of Medical Instrumentation issued the following news release:
In a move with potential implications for makers of medical devices, DuPont is changing the manufacturing process for Tyvek, a high-density, polyethylene material widely used in the packaging of such devices.
DuPont says it's working with the U.S. Food and Drug Administration to ensure the transition does not complicate the clearance process for medical devices.
In a document explaining the transition, the company says its analysis of the new manufacturing process shows that the Tyvek made the new way "does not represent a significant change in functional performance."
If the FDA agrees, "it would issue guidance indicating that medical devices manufacturers would not be required to file amended 510(k)s or premarket approvals (PMAs) for existing devices because the transition represents a merge, or lot, change," according to DuPont.
Over the course of the transition, the FDA will review data generated by third parties which will show whether the new Tyvek is "functionally equivalent."
DuPont is transitioning the Tyvek 1073B and the 1059B styles to new manufacturing lines that use a flash-spinning technology, which the company says allows for greater capacity.
The Tyvek Asuron and Tyvek 2FS styles won't be affected by this as they are already manufactured using this technology.
DuPont says it also shared the transition plan with global regulatory authorities and expects "they will take the same position as the FDA."
The company says it expects the new Tyvek to be available by late 2012 for new devices and in 2014 for existing devices.
For additional information on the transition from DuPont, click here
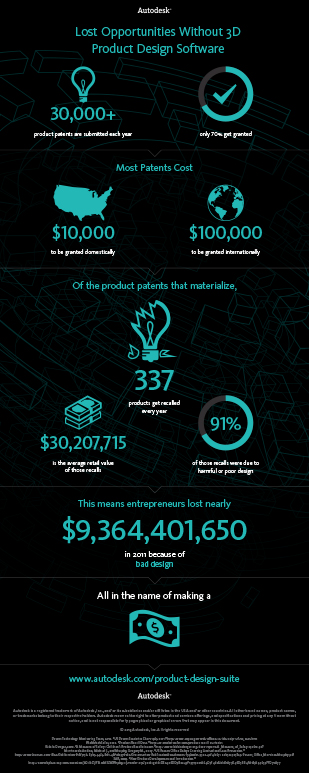
Simulate to Innovate and Save
Innovate & Save Money don't pay the Price for Bad Design
The drive to reduce time to market and increase the speed of innovation increases risk and can lead to defects in products. The importance of getting to market fast is well established however, accuracy, minimum risk, and without flaws, are powerful needs that when met lead to large cost savings. Defects detected by end users of your products cause not only financial loss but damage to your brand as well.
AUTODESK® recently published data on the costs associated with "Bad Design". The below infographic is based on 2011 statistics where AUTODESK® explores how bad design leads to lost opportunities. 3D product design software gives you the ability to design, visualize, and simulate your product virtually, which leads to cost savings and better designed products.
While there are many tools available for 3D product design and virtual product development they are only a part of the process. To fully realize the benefits of these tools they must be coupled with a depth and breadth of experience in product design and engineering. Tools are only as good as the person using them. Skilled and experienced engineers produce greater insight into the solution space for the answers to difficult questions. These insights lead to knowledge that can be leveraged over time, from idea to sales, building brands that matter. As AUTODESK® points out there is a lot of money wasted on bad designs. But if true savings are to be achieved an investment in tools is not enough. The right tools, in the right hands, leads to the right answers that achieve multiple benefits throughout the product development process.
Customer Benefits of Virtual Product Development
- Better Products
- Lower Risk
- Reduce Time to Market
- Eliminate Errors
- Saving You Money
SGI Acquires OpenCFD Ltd
OpenCFD Ltd. has announced it will be acquired by SGI corporation. Select the Read more link to see their announcement and for links to information about OpenFOAM and GNU.
CDRH 2014 Proposed Guidance Development
The U.S. Food and Drug Administration’s (FDA) Center for Devices and Radiological Health (CDRH) has released its fiscal year 2014 plans for guidance development.
Prioritized Areas Of Focus Include the following Final and Draft Topics:
Final Guidance Topics
- Center for Devices and Radiological Health Appeals Processes: Questions and Answers About 517A
- Content of Premarket Submissions for Management of Cybersecurity in Medical Devices
- Providing Information about Pediatric Uses of Medical Devices Under Section 515A of the Federal Food, Drug, and Cosmetic Act
- De Novo Classification Process (Evaluation of Automatic Class III Designation)
- The Pre-Submission Program and Meetings with FDA Staff
- The 510(k) Program: Evaluating Substantial Equivalence in Premarket Notifications
- Types of Communication During the Review of Medical Device Submissions
- Premarket Notification [510(k)] Submissions for Medical Devices that Include Antimicrobial Agents
- Applying Human Factors and Usability Engineering to Optimize Medical Device Design
- In Vitro Companion Diagnostic Devices
- Global Unique Device Identification Database
- Design Considerations for Pivotal Clinical Investigations for Medical Devices
Draft Guidance Topics
- Benefit-Risk Determinations in Premarket Notifications (510(k)s)
- Appropriate Use of Voluntary Consensus Standards in Premarket Submissions
- Custom Devices
- Hearing Aids and Personal Sound Amplification Products (PSAPs)
FDA Proposes International Consortium of Cardiovascular Registries
Proposal for Cardiovascular Registries
The FDA filed a Notice of Public Meeting and Request for Comments regarding what it is calling the "International Consortium of Cardiovascular Registries." The purpose is to discuss the development of the registry and to garner feedback from interested parties on the following topics:
- The role of registry consortia in postmarket surveillance
- Goals of the International Consortium of Cardiovascular Registries,
- Lessons learned from the development of the ICOR,
- Development of an international consortium of transcatheter valve registries as a pilot phase,
- Analysis of near- and long-term outcomes reported through registries, and
- Discussion of capabilities, challenges, and limitations of existing transcatheter valve registries.
The FDA indicates that the effort will be modeled on the International Consortium of Orthopedic Registries (ICOR) and will begin with transcatheter valve therapy devices and procedures.
The The meeting will be held on April 22, 2013, from 8 a.m. to 5 p.m. at FDA's White Oak Campus, 10903 New Hampshire Ave., Bldg. 31 Conference Center, the Great Room (Rm. 1503), Silver Spring, MD 20993-0002.
FDA Plans More Interactive Reviews
FDA Seeks Increased Efficiency of Review Process
The Food and Drug Administration (FDA) has issued Draft Guidance for Industry and Food and Drug Administration Staff regarding the types of Communication During the Review of Medical Device Submissions. The FDA is seeking feedback on the proposed guidance rules. This guidance updates the Agency's approach to Interactive Review. It now reflects the FDA's implementation of the Medical Device User Fee Act of 2007 (MDUFA II) Commitment Letters and of undertakings agreed in connection with the Medical Device User Fee Amendments of 2012 (MDUFA III). It also incorporates additional types of communication, all of which are designed to increase the efficiency of the review process. (Download PDF)
The concept of the Interactive Review was discussed in detail as part of the Medical Device User Fee Act (MDUFA) II of 2007. The process was further described in the guidance “Interactive Review for Medical Device Submissions: 510(k)s, Original PMAs, PMA Supplements, Original BLAs, and BLA Supplements.” (Download PDF)
Additional funds obtained from user fees will enable the FDA, with the cooperation of industry, to improve the device review process. The FDA seeks to implement improvements for the medical device review process that will provide further transparency into the review process. Improvements include new communication commitments. These additional communications are in the context of: acceptance review; substantive interactions; and, if applicable, missed MDUFA goals.
This guidance describes four types of communication during the review of a medical device submission:
- Acceptance Review Communication for premarket notification submissions (510(k)s), original premarket approval applications (Original PMAs), and Panel-Track PMA Supplements
- Substantive Interaction for 510(k)s, Original PMAs, Panel-Track PMA Supplements, and 180-Day PMA Supplements;
- Interactive Review for 510(k)s, Original PMAs, PMA Supplements, original Biologics License Applications (Original BLAs), and BLA Supplements
- Missed MDUFA Decision Communication for 510(k)s, Original PMAs, and Panel-Track PMA Supplements
Through the Interactive Review process the FDA seeks to facilitate efficient and timely review of premarket submissions. Increased informal interaction between FDA and applicants will include the exchange of scientific and regulatory information.
The Interactive Review process is designed to help accomplish the following:
- Improve the interaction between the FDA review staff and the applicant during the review process;
- Prevent unnecessary delays in the completion of the review, thus reducing the overall time to market;
- Ensure that FDA’s concerns are clearly communicated to the applicant during the review process, as appropriate;
- Minimize the number of review cycles;
- Minimize the number of review questions conveyed through deficiency letters; and
- Ensure timely responses from applicants.
- Interactive Review has no start/stop impact on the review clock.
Types of Deficiencies Appropriate for Interactive Review
More significant than “minor,” but that can likely be addressed by the applicant in a time frame that would allow FDA review of the response prior to the MDUFA performance goal for that submission type without placing the submission on hold.
Examples include, but are not limited to:
- Requests for limited additional short-term laboratory bench or biocompatibility testing
- Further justification for the omission of a test
- Additional statistical analysis of the clinical data not related to the primary safety or effectiveness endpoint
Timing of Interactive Review
Interactive Review After Substantive Interaction for 510(k)s, Original PMAs, Panel-Track PMA Supplements, and 180-Day PMA Supplements
Any new deficiencies (i.e., deficiencies not raised as part of the Substantive Interaction) should be limited to issues raised by the information provided by the applicant in its response, unless the reviewer concludes (and received supervisory concurrence) that the initial deficiencies identified do not adequately address important issues materially relevant to a decision of substantial equivalence (510(k)) or safety and effectiveness (PMA).19 For example, following the communication of deficiencies in a 510(k) AI letter, FDA might become aware of a heightened potential for device failure through a series of recalls on other devices with a similar feature. If these recalls indicate that the particular bench test performed by the applicant to evaluate this feature is not predictive of clinical performance, an FDA reviewer, with appropriate supervisory concurrence, might request additional testing to address the safety of this feature to determine substantial equivalence. As the end of the review cycle approaches, FDA intends to send a communication that lists the remaining issues, limiting the applicant’s response timeframe to a maximum of 7 calendar days and allowing time for FDA to review the response, so that a timely MDUFA decision can be made.
In limited circumstances, a second AI letter for a 510(k) may be appropriate. One example of such a circumstance would be when a first AI letter indicates that FDA believes no predicate device exists, but the submitter is able to identify a predicate. A subsequent review of the comparison of the subject device to the newly identified predicate could raise questions appropriate for a second AI request. Other instances in which a second AI request could be issued should be limited and occur only with concurrence of Division-level management.
Additional Interactive Review
The FDA also encourages the use of Interactive Review at other points in the review process to facilitate the efficient and timely review of a submission. At FDA’s discretion, Interactive Review can be used:
- Prior to Substantive Interaction for 510(k)s, Original PMAs, Panel-Track PMA Supplements, and 180-Day PMA Supplements; and
- As needed for BLAs, BLA supplements, Humanitarian Device Exemptions (HDEs), and Product Development Protocols (PDPs).
FDA should determine an acceptable timeframe for the applicant to provide a response to the deficiencies based on MDUFA, Office, or Center timelines. The established timeframe should be based on the impending review deadline, the estimated time that the applicant should need to respond, and the estimated time that FDA should need to review the response.
When final, this document will supersede "Interactive Review for Medical Device Submissions: 510(k)s, Original PMAs, PMA Supplements, Original BLAs, and BLA Supplements" dated February 28, 2008. If interested in providing comments regarding this draft they can be submitted HERE
FDA Extreme Weather Effects on Medical Device Safety and Quality
The Food and Drug Administration, HHS request for comments on effects of extreme weather on medical device safety and quality.
The Food and Drug Administration (FDA) is studying the potential effects of extreme weather and natural disasters on medical device safety and quality. FDA is announcing at this time its request for comments on the topic of extreme weather effects on medical device safety and quality. Submit either electronic or written comments by May 10, 2013. Submit electronic comments to http:// www.regulations.gov. Submit written comments to the Division of Dockets Management (HFA–305), Food and Drug Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD 20852. Identify comments with the docket number found in brackets in the heading of this document.
FDA is seeking information particularly on the following questions; however, you may respond to any, all, or none of these questions, or you may submit comments on any topic relating to the purposes of this document, regardless of whether a topic is addressed by these questions:
- Have you experienced any of the scenarios or any other effects of EW on the safety and effectiveness of medical devices?
- How did you respond to extended periods of electrical or network outages or other events related to EW?
- In past EW situations, how was communication handled between the manufacturer facility and patients/users about the safe use of products during EW events? How did you provide/ receive information about device failures? Do you have any suggestions for complaint handling during these situations?
- How should industry optimize the design, production, and use of medical devices during and after EW events?
- How could products be monitored during transport and storage in light of potential interruptions and environmental extremes from EW events?
- How can manufacturers best prevent or minimize temporary shortages of medical devices when EW may damage existing inventory or impact just-in-time production of critical components?
- In what ways have EW events impacted your manufacturing site? What were the lessons learned during the recovery process as you returned to production? What changes were made as a result of the EW event?
- Are there additional steps FDA can take to help industry anticipate, mitigate, or better tolerate the effects of EW?
- Are there steps that standards development or other professional organizations can take to support industry to optimally prepare for EW events?
FOR FURTHER INFORMATION CONTACT: Jennifer Kelly, Center for Devices and Radiological Health, Food and Drug Administration, 10903 New Hampshire Ave., Bldg. 66, rm. 3429, Silver Spring, MD 20993–0002, This email address is being protected from spambots. You need JavaScript enabled to view it. (more)
FDA Proposes Amendments on Clinical Data Acceptance
FDA New Proposed Regulations for Clinical Data Submission
The Food and Drug Administration (FDA) is proposing to amend its regulations on acceptance of data from clinical studies for medical devices. They are proposing new requirements for clinical studies conducted outside the United States as support for an investigational device exemption (IDE) application, a premarket notification (510(k)) submission, a premarket approval (PMA) application, a product development protocol (PDP) application, or a humanitarian device exemption (HDE) application to be conducted in accordance with good clinical practice (GCP). This will include obtaining and documenting the review and approval of the study by an independent ethics committee (IEC) and obtaining and documenting freely given informed consent of study subjects.
Their intent is to update the FDA standards for acceptance of data from clinical studies conducted outside the United States and to help ensure the protection of human subjects and the quality and integrity of data obtained from these studies. The FDA is also proposing amendments to the IDE and 510(k) regulations to address the requirements for FDA acceptance of data from clinical studies conducted inside the United States.
The proposed amendments are intended to provide consistency in FDA requirements for acceptance of clinical data, whatever the application or submission type. Note that comments are due by May 28, 2013
Link to PROPOSED RULE
FDA Final Ruling Good Manufacturing Requirements for Combination Products
Good Manufacturing Practice Requirements for Combination Products
Earlier this week the FDA released its Final Rules regarding Good Manufacturing Practice Requirements for Combination Products. The Food and Drug Administration (FDA or Agency) is issuing this regulation on the current good manufacturing practice (CGMP) requirements applicable to combination products. This rule is intended to promote the public health by clarifying which CGMP requirements apply when drugs, devices, and biological products are combined to create combination products. In addition, the rule sets forth a transparent and streamlined regulatory framework for firms to use when demonstrating compliance with CGMP requirements for “single-entity” and “co-packaged” combination products.
Products Affected Include
- Existing Combination Products
- The FDA will provide help for manufacturers to alter systems as need be for existing products
- New Combination Products
- Component manufacturers are exempt from the CGMP rules even if a component will be incorporated into the final product.
More Information
Combination Product Definition
Combination products are defined in 21 CFR 3.2(e). The term combination product includes:
- A product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are physically, chemically, or otherwise combined or mixed and produced as a single entity;
- Two or more separate products packaged together in a single package or as a unit and comprised of drug and device products, device and biological products, or biological and drug products;
- A drug, device, or biological product packaged separately that according to its investigational plan or proposed labeling is intended for use only with an approved individually specified drug, device, or biological product where both are required to achieve the intended use, indication, or effect and where upon approval of the proposed product the labeling of the approved product would need to be changed, e.g., to reflect a change in intended use, dosage form, strength, route of administration, or significant change in dose; or
- Any investigational drug, device, or biological product packaged separately that according to its proposed labeling is for use only with another individually specified investigational drug, device, or biological product where both are required to achieve the intended use, indication, or effect.
Russian Medical Device Regulations 2013
The Russian Federation has a list of slated changes to its Medical Device Regulations for 2013. Final implementation of the rules changes is still pending. The Emergo Group discussed the changes with the Russian Ministry of Health and Roszdravnadzor officials and they listed the following potential changes for 2013:
- Applicant must obtain an import permit for samples submitted for testing by Rozsdravnadzor.
- Applicant must establish agreements with laboratories for any necessary technical and toxicological testing of its device.
- Applicant must submit documentation including test reports to Roszdravnadzor.
- Roszdravnadzor authorizes expert review of the applicant’s submission.
- Expert reviewers determine whether additional clinical testing of device is necessary and provides list of hospitals where such tests should occur.
- Roszdravnadzor informs applicant whether clinical tests are required.
- Applicant must set up agreements with hospitals where clinical testing will take place.
- Applicant provides clinical testing results to Roszdravnadzor.
- Roszdravnadzor then sends applicant’s clinical test results out for expert review.
- Expert reviewers notify Roszdravnadzor whether clinical test results are acceptable, provide a report of the test review to regulators, and inform Roszdravnadzor whether the device in question can be registered.
- Roszdravnadzor issues either a Registration Certificate to applicant based on a positive expert review or a refusal based on a negative review.
More Information can be found at the Emergo Group Website
Medical Device Tax Final Regulations
Medical Device Tax
The Internal Revenue Service (IRS) has issued the Medical Device Tax Final Regulations
The United States Internal Revenue Service (IRS) has released a finalized guidance report on the upcoming 2.3 percent medical device tax. The new tax will become active in January of 2013.
The IRS released a 58-page report on how it will enforce and regulate the upcoming tax. According to the report, the IRS will define a taxable medical device as any device listed with the United States Food and Drug Administration (FDA) under sec. 510(j) of its FFDCA and under 21 CFR pt. 807.
If a device is not listed with the FDA under the regulations listed above, it will not be taxed. However, a device may be taxed if the FDA determines that device should have been listed with the agency. In this situation, the manufacturer of the medical device will be required to pay the 2.3 percent tax starting on the date it was notified of the corrective action by the FDA.
Many medical device companies have publicly stated that they oppose the tax. They argue that the 2.3 percent medical device tax will discourage innovation. In addition, the new tax has been scrutinized due to the way it taxes a company’s income. Since the medical device tax is levied on revenue instead of profit, it may have a disproportionately negative impact on startups and other new medical device manufacturers.
http://www.qmed.com/news/final-guidance-upcoming-medical-device-tax-released-irs?cid=nl_qmed_daily_europe
Innovation Pathway, the Future of working with the FDA
FDA Innovation Pathway
In order to get patients faster access to safe and effective medical devices that address unmet public health needs, the FDA is developing what it calls the "Innovation Pathway." The Innovation Pathway will be a new way of doing business within the FDA's existing regulatory framework. The initiative is particularly focused on breakthrough technology.
The primary goal for the new Innovation Pathway is to shorten the overall time and cost it takes for the development, assessment and review of medical devices. The FDA also seeks to improve how FDA staff and innovators work together. Engaging with innovators much earlier, more collaboratively, and in new ways, should help to reduce the time and cost of the entire process.
On April 9, 2012, the FDA's Center for Devices and Radiological Health (CDRH) launched its second version of the Innovation Pathway, called "Innovation Pathway 2.0." According to the FDA, Innovation Pathway 2.0 offers new and modified tools and methods to deepen collaboration between the FDA and innovators early in the process, prior to pre-market submission, with the goal of making the regulatory process more efficient and timely.
The Pathway also serves as a living laboratory to test new tools and methods for breakthrough devices that we may also apply to other technologies to enhance all of our device pre-market programs.
CLICK HERE FOR MORE INFORMATION
FDA Unique Device Identification
FDA Unique Device Identification
The Food and Drug Administration (FDA) released a proposed the "unique device identifier" rule requiring that most medical devices distributed in the United States carry a unique device identifier, or UDI. A UDI system is designed to improve the quality of information in medical device adverse event reports. This is intended to help the FDA identify product problems more quickly and allow better targeted recalls that will improve patient safety.
Senate Approves FDA User Fee Bill
United States Senate voted 92-4 passing User Fee Bill
Tuesday June 26th, 2012 the United States Senate has voted 92-4 passing the FDA User Fee Bill and sending it on for Presidential signature.
News and Reactions:
Kaiser Health News: Senate Sends FDA User Fee Bill To Obama; House GOP Putting Pressure On AARP
http://www.kaiserhealthnews.org/Home/Daily-Reports/2012/June/27/capitol-hill-watch.aspx
Mass Device: AdvaMed: MDUFMA III "isn't your father's user fee agreement"
http://www.massdevice.com/news/advamed-mdufma-iii-isnt-your-fathers-user-fee-agreement?page=show
QMED: Boston Scientific Praises Passage of User Fee Bill
http://www.qmed.com/news/boston-scientific-praises-passage-user-fee-bill
MDDI Online: Senate Overwhelmingly Passes Unprecedented Medical Device User Fee Act
http://www.mddionline.com/article/senate-overwhelmingly-passes-medical-device-user-fee-act
Small Companies Effected most by FDA's 510k Process
Small Companies Facing FDA Issues
A recent report in the June issue of Journal of Medical Devices (Article) highlights the issues facing small companies that deal with the FDA to get medical devices approved. The study found that predictability of the regulatory process in the United States is a key priority given that two-thirds of the medical device companies surveyed listed this issue as "critically important."
FDA Proposed Pilot Triage Program
FDA Internal Pilot Program for 510k Review
The FDA is running a pilot program, April 2nd to October 2nd 2012, for review of 510(k) applications. It is an internal program designed with the following objectives:
- Two Tiered Review
- Quick Review Tier
- Good Quality submissions can clear as soon as possible but within 30 days
- Must pass Quick Review Criteria
- Pass a Total Product Life Cycle (Postmarket) Search
- Seek clearance for a device for which FDA has review experience and knowledge of expected performance
- Not need an extensive consult to complete 510(k) review
- Contain a 510(k) Summary and not a 510(k) Statement
- Regular Review Tier
- Normal 510(k) review, clear within 90 days
- Quick Review Tier
- Reduce the review time of Traditional 510(k) applications that are of good quality
- Creates an incentive to sponsors to submit good quality applications to OIVD
- Diminish the effort and time reviewers dedicate to Traditional 510(k) applications for products that are well known to the Office
- Attain faster product availability
- Increase reviewer time for other work-related activities such as training/continuing education, or other regulatory activities

For more: PROPOSED PILOT TRIAGE PROGRAM
FDA Proposes Medical Device Guidelines
Draft Guidance from FDA on Benefit-Risk
For the first time, the U.S. Food and Drug Administration has provided draft guidance clarifying how benefit-risk determinations are made during pre-market review of certain medical devices.
FDA Seeks Public Comments - IOM Recommendations
FDA Seeking Public Comments on 510k Program
In a Press Release the FDA announced that it will open a public docket to begin receiving public comments on the Institute of Medicine's - IOM - report on the 510k program, the most common pathway to market for lower-risk medical devices.
IOM Releases CDRH 510k Study
Institute of Medicine releases study of the FDA 510k process
The Institute of Medicine - IOM - released its study of the FDA CDRH 510k process for Medical Device approval. The committee recommends that the FDA should invest in developing a new regulatory framework to replace the, quote, flawed 510k medical device clearance process. They cite that the FDA's limited legal power and resources cause the 510k process to be and unreliable screen for safety and effectiveness of moderate-risk Class II devices.
FDA Industry Guidance - 510k Modifications
Draft FDA Guidance for 510k device changes
FDA's Center for Devices and Radiological Health announced the release of the draft of the FDA's guidance for 510k device changes. Jeffrey Shuren, M.D., director of FDA's CDRH, stated that "We are making the regulatory process for medical devices less challenging by better describing our expectations." "In particular, manufacturers can continue to make innovative improvements to their devices and better plan for any updated submissions. This saves time and money."
Improvements Strengthening CDRH
FDA Seeks to "Strengthen" CDRH
The FDA Center for Devices and Radiological Health - CDRH - continues it's efforts to, as it states, strengthen CDRH and increase our ability to protect and promote the public health through the implementation of changes and improvements. CDRH 2011 Strategic Priorities PDF
Patents and Trademarks Encourage New Technology
Legislation to Protect USPTO from Sequestration
Three Congressional Members from California, Honda, Lofgren, and Eshoo, have proposed legislation to protect the United States Patent Office (USPTO) from sequestration. The Act is designated as the "Patents and Trademarks Encourage New Technology Jobs Act" or "PATENT Jobs Act" for short. In summary the bill would protect the USPTO from being subject to sequester cuts currently requiring $150 million in the agency's funding.
The USPTO is entirely funded by fees paid by patent filers as such it does not fall into the same government spending as other areas of government. These fees are used solely to carry out USPTO operations hence they should not be appropriated for other areas of government. This bill would prevent sequestration of USPTO revenues.
The bill found bipartisan support from members of the California delegation to the Commerce, Justice, and Science Appropriations Subcommittee, they submitted a bipartisan letter to the committee: LETTER
Congressional Representative Mike Honda comments: LINK
The Shield Act and Thoughts on Patent Trolls
Of Trolls and Treasures
The House Judiciary committee held hearings last week (3/13/2013) regarding comments on the new "Shield Act". The Shield Act is designed to address so called "Patent Trolls" and protect, or as the bill states "Saving High-Tech Innovators" from the ugly ole' trolls. In a recent article we presented a perspective on the issue HERE and further commentary on the details of the basis upon which the new "Shield Act" has been written, in short, it is based upon biased and "secret data" as provided in a recent article by Adam Mossoff at Truth on The Market HERE. Mossoff indicates that the $29 Billion price tag given for "Egregious Legal Disputes" was from a "secret survey" done by RPX, a company that makes its money by defending companies against "Patent Trolls." Not exactly scientific and along with that neither the data nor the study has been published.
At the base of all of this is the definition of a Patent as "Property". If an inventor, large or small, has an issued patent then by law it is "Intellectual Property" and the owner has rights and the ability under the law to enforce those rights. Some will point to "bad patents" in connection with "Patent Trolls" however one has nothing to do with the other. If a patent is "bad" or invalid that should be addressed through the normal course of the patent prosecution at the USPTO and there are already measures in place for those issues. However, one cannot simply say that because an inventor is deemed a "Non-Practicing-Entity" (NPE), i.e. a "Patent Troll", that they are unworthy of ownership rights by rhetoric alone. This is a slippery slope that if allowed to continue may render patents worthless and truly stifle innovation. We pointed this out in another article HERE showing just how strange some logic can get when Google simply states that Apple should simply give its property away because they are just too successful! Isn't that the point, to innovate and be successful?
Judiciary Hearing on Litigation Abuse and Patent Trolls
SHIELD ACT vs PATENT TROLLS
HR 845 Saving High-Tech Innovators from Egregious Legal Disputes
The U.S. House of Representatives has introduced H.R. 845 (Ref HR 6245 of 112th Congress) known as the "Saving High-Tech Innovators from Egregious Legal Disputes Act of 2013" designed to address the "Patent Troll" issue relating to frivolous law patent infringement law suits. The amendment to chapter 29 of title 35, United States Code, is designed to provide for the recovery of patent litigation costs, and for other purposes. The text of the of the bill is provided below and a PDF of the bill can be downloaded HERE.
[Congressional Bills 113th Congress]
[From the U.S. Government Printing Office]
[H.R. 845 Introduced in House (IH)]
113th CONGRESS
1st Session
H. R. 845
To amend chapter 29 of title 35, United States Code, to provide for the
recovery of patent litigation costs, and for other purposes.
_______________________________________________________________________
IN THE HOUSE OF REPRESENTATIVES
February 27, 2013
Mr. DeFazio (for himself and Mr. Chaffetz) introduced the following
bill; which was referred to the Committee on the Judiciary
_______________________________________________________________________
A BILL
To amend chapter 29 of title 35, United States Code, to provide for the
recovery of patent litigation costs, and for other purposes.
Be it enacted by the Senate and House of Representatives of the
United States of America in Congress assembled,
SECTION 1. SHORT TITLE.
This Act may be cited as the ``Saving High-Tech Innovators from
Egregious Legal Disputes Act of 2013''.
SEC. 2. RECOVERY OF LITIGATION COSTS.
(a) Amendment.--Chapter 29 of title 35, United States Code, is
amended by inserting after section 285 the following new section:
``Sec. 285A. Recovery of litigation costs
``(a) In General.--In an action involving the validity or
infringement of a patent--
``(1) a party asserting invalidity or noninfringement may
move for judgment that the adverse party does not meet at least
one of the conditions described in subsection (d);
``(2) not later than 90 days after a party has moved for
the judgment described in paragraph (1), the adverse party
shall be provided an opportunity to prove such party meets at
least one of the conditions described in subsection (d);
``(3) as soon as practicable after the adverse party has
been provided an opportunity to respond under paragraph (2),
but not later than 120 days after a party has moved for the
judgment described in paragraph (1), the court shall make a
determination whether the adverse party meets at least one of
the conditions described in subsection (d); and
``(4) notwithstanding section 285, the Court shall award
the recovery of full costs to any prevailing party asserting
invalidity or noninfringement, including reasonable attorney's
fees, other than the United States, upon the entry of a final
judgment if the court determines that the adverse party did not
meet at least one of the conditions described in subsection
(d), unless the court finds that exceptional circumstances make
an award unjust.
``(b) Bond Required.--Any party that fails to meet a condition
under subsection (a)(3) shall be required to post a bond in an amount
determined by the court to cover the recovery of full costs described
in subsection (a)(4).
``(c) Timing and Effect of Pending Motion.--With respect to any
motion made pursuant to subsection (a)(1) the following applies:
``(1) In the case of a motion that is filed before the
moving party's initial disclosure are due--
``(A) the court shall limit any discovery to
discovery that is necessary for the disposition of the
motion; and
``(B) the court may delay issuing any scheduling
order until after ruling on the motion.
``(2) In the case of a motion that is filed after the
moving party's initial disclosures are due the court may delay
ruling on the motion until after the entry of final judgment.
``(3) In the case of a motion that is filed after the entry
of final judgment, any such motion must be combined with a
motion for fees to the prevailing party.
``(d) Condition Defined.--For purposes of this section, a
`condition' means, with respect to the party alleging infringement, any
of the following:
``(1) Original inventor.--Such party is the inventor, a
joint inventor, or in the case of a patent filed by and awarded
to an assignee of the original inventor or joint inventor, the
original assignee of the patent.
``(2) Exploitation of the patent.--Such party can provide
documentation to the court of substantial investment made by
such party in the exploitation of the patent through production
or sale of an item covered by the patent.
``(3) University or technology transfer organization.--Such
party is--
``(A) an institution of higher education (as that
term is defined in section 101 of the Higher Education
Act of 1965 (20 U.S.C. 1001); or
``(B) a technology transfer organization whose
primary purpose is to facilitate the commercialization
of technology developed by one or more institutions of
higher education.''.
(b) Technical and Conforming Amendment.--The table of sections for
chapter 29 of title 35, United States Code, is amended by inserting
after the item relating to section 285 the following new item:
``285A. Recovery of litigation costs for patent.''.
(c) Effective Date.--The amendment made by subsection (a) shall
take effect on the date of the enactment of this Act and shall apply to
any action involving the validity or infringement of a patent for which
a complaint is filed on or after the date of the enactment of this Act.
USPTO Publishes Final Rules and Guidelines Governing First-Inventor-to-File
The U.S. Department of Commerce’s United States Patent and Trademark Office (USPTO) today published final rules of practice implementing the first-inventor-to-file provision of the Leahy-Smith America Invents Act (AIA). The provision, one of the hallmarks of the AIA, is a major step towards harmonization of the U.S. patent system with those of the United States’ major trading partners, allowing greater consistency in the prosecution and enforcement of U.S. patents. The AIA also includes safeguards to ensure that only an original inventor or his assignee may be awarded a patent under the first-inventor-to-file system. The first-inventor-to-file provision of the AIA goes into effect on March 16, 2013, and represents the final implementation of the changes mandated by the AIA.
Read more: http://www.uspto.gov/news/pr/2013/13-10.jsp
USPTO New Fee Schedule
New Patent Fees are scheduled to become effective on March 19, 2013
The United States Patent and Trademark Office (Office or USPTO) sets or adjusts patent fees in this rulemaking as authorized by Section 10 of the Leahy-Smith America Invents Act (Act or AIA). Section 10 prescribes that fees may be set or adjusted only to recover the aggregate estimated costs to the Office for processing, activities, services, and materials relating to patents, including administrative costs to the Office with respect to such patent operations. Section 10 authority includes flexibility to set individual fees in a way that furthers key policy considerations, while taking into account the cost of the respective services. See Section 10 of the Act, Public Law 112-29, 125 Stat. at 316-17. Section 10 also establishes certain procedural requirements for setting or adjusting fee regulations, such as public hearings and input from the Patent Public Advisory Committee and oversight by Congress.
The fees will provide the Office with a sufficient amount of aggregate revenue to recover its aggregate cost of patent operations, while helping the Office implement a sustainable funding model, reduce the current patent application backlog, decrease patent application pendency, improve patent quality, and upgrade the Office's patent business information technology (IT) capability and infrastructure.
This final rule sets or adjusts 351 patent fee where 93 apply to large entities, 94 to small entities, 93 to micro entities, and 71 are not entity-specific. Also, despite increases in some fees, applicants who meet the new micro entity definition will pay less than the amount paid for small entity fees under the current fee schedule for 87 percent of the fees eligible for a discount under section 10(b).
Another stated objective of the fee setting is to encourage innovators to take advantage of patent protection, the Office sets basic ‘‘front-end’’ fees (e.g., filing, search, and examination) below the actual cost of carrying out these activities. The Office provides fee reductions for small and micro entity innovators to facilitate access to the patent system. Setting front-end and small and micro entity fees below cost requires, however, that other fees be set above cost. To that end, the Office sets basic ‘‘back-end’’ fees (e.g., issue and maintenance) in excess of costs to recoup revenue not collected by front-end and small and micro entity fees. Charging higher back-end fees also fosters innovation and benefits the overall patent system. After a patent is granted, a patent owner is better positioned, as opposed to at the time of filing a patent application, to more closely assess the expected value of an invention, which is a consideration in determining whether to pay maintenance fees to keep the patent protecting the invention in force. Expiration of a patent makes the subject matter of the patent available in the public domain for subsequent commercialization. Determining the appropriate balance between front-end and back-end fees is a critical component of aligning the Office’s costs and revenues.
Summary of New Fee Structure Includes:
See full comparison of Fee Changes Here: USPTO-Fees
- Micro Entity Fee: 75% reduction of fees set under Section 10(a) including filing, searching, examining, issuing, appealing, and maintaining patent applications and patents. (universities qualify as Micro Entity)
- Small Entity Fee: 50% reduction of fees set under Section 10(a) including filing, searching, examining, issuing, appealing, and maintaining patent applications and patents.
- Issue Fees: $960 (Note: $300 publication fee eliminated) However, these fee changes will not be implemented until January 2014
- Maintenance Fees:
- First Fee at 3 ½ years: $1,150 to $1,600.
- Second Fee at 7 ½ years: $2,900 to $3,600.
- Third Fee at 11 ½ years: $4,810 to $7,400.
- Claim Fees:
- Greater than 3 Independent Claims: $420 per claim
- Greater than 20 Total Claims: $80 per claim
- RCE Fees: $1,200 along with a surcharge of $500 for as second RCE filing
- Appeals: New surcharge of $2,000 for forwarding the brief to the PTAB rather than settling with the examiner.
- Ex Parte Reexamination: $12,000, small and micro entity privileges apply
- Inter Partes Reviews: $23,000 with a $14,000 refund if IPR petition is rejected
- Post Grant Reviews: $30,000 with a $18,000 refund if IPR petition is rejected
- Derivation Petition: $400
- Assignments: Free if submitted electronically
Design patent fees are also changing:
- Total Filing Fees: $760
- Issue Fee: $560
Details from the Federal Register: Link
HR 6621 Signed into Law
On January 14th, 2013 H.R. 6621, "An Act to correct and improve certain provisions of the Leahy-Smith America Invents Act and title 35, United States Code," was signed into law.
Some News and Reactions
http://www.patentlyo.com/patent/2013/01/aia-technical-amendment-becomes-law.html
Read the Bill:
http://www.gpo.gov/fdsys/pkg/BILLS-112hr6621enr/pdf/BILLS-112hr6621enr.pdf
H.R. 6621 Passes House
Corrections to the Leahy-Smith America Invents Act and title 35, United States Code
112th Congress, 2nd Session H.R. 6621
H.R. 6621 was introduced to make corrections to certain provisions of the Leahy-Smith "America Invents Act" (AIA) and it passed the U.S. House of Representatives by a vote of 308-89.
Silence is Golden
First Inventor to File means Keep Quiet!
We have highlighted the patent process and new changes to it via the AIA (America Invents Act) and some of the effects it can have on small entities. Though the patent process is evolving and complex there is one important thing that can be done by any inventor, big or small, and that is to keep your ideas quiet.
Now, given that we have transitioned to a First-Inventor-to-File (FItF) system like the rest of the world, along with other changes we won't detail here, silence truly has become golden. The following article indicates that big things can come from simple things like an overheard conversation. It involves the case of Dr. Warren Selman, who overheard a conversation between two Israel Air Force officers one day.
Granted, the outcome in this case was positive for those involved. However, one need not think too hard to see that casual chatter about your idea can give others, who may not engage with you like Dr. Selman did in this case, the chance to develop the idea or at the very least a competitive idea by themselves simply by beating you to the patent office!
Overheard in a coffee shop: A better way to do brain surgery, based on Israeli simulation technology
Do Patents hold any meaning anymore
Google Says, "Apple needs to Share!"
Is Google going to hand over it's proprietary search algorithms due to it's more than ubiquitous use by billions of people around the globe? This is strange logic. How can a large company such as Google think it can simply state that another companies hard work and investment should be considered as free to those who wish to use it? This is a disturbing trend and one with no small level of foretelling from the America Invents Act. Is it possible that corporations such as Google have been emboldened by the large company leanings of the AIA? There is a clear favorite in the race to innovate in regards to the AIA and it is not the small inventor.
In a letter to the Senate Judiciary Committee, Google General Counsel Kent Walker wrote:
While collaborative [Standards Setting Organizations (SSOs)] play an important part in the overall standard setting system, and are particularly prominent in industries such as telecommunications, they are not the only source of standards. Indeed, many of the same interoperability benefits that the FTC and others have touted in the SSO context also occur when one firm publishes information about an otherwise proprietary standard and other firms then independently decide (whether by choice or of necessity) to make complementary investments to support that standard in their products. … Because proprietary or de facto standards can have just as important effects on consumer welfare, the Committee’s concern regarding the abuse of SEPs should encompass them as well.
As Sewell writes:
The capabilities of an iPhone are categorically different from a conventional phone, and result from Apple’s ability to bring its traditional innovation in computing to the mobile market. Using an iPhone to take photos, manage a home-finance spreadsheet, play video games, or run countless other applications has nothing to do with standardized protocols. Apple spent billions in research and development to create the iPhone, and third party software developers have spent billions more to develop applications that run on it. The price of an iPhone reflects the value of these nonstandardized technologies — as well as the value of the aesthetic design of the iPhone, which also reflects immense study and development by Apple, and which is entirely unrelated to standards.
NTP gets it right.
Patent licensing company NTP, best known for a big settlement with Research In Motion, has reached a deal with most of the rest of the wireless industry.
NTP said Monday it “has reached a mutual resolution with 13 companies,” including all four major U.S. cell phone carriers; device makers including Apple, HTC, Motorola, Samsung and LG; as well as Google, Microsoft and Yahoo. NTP sued the carriers in 2007 and and filed more suits in 2010.
Details on the pact are fuzzy, with a press release from NTP not even saying that the company is getting money, let alone how much. NTP got more than $600 million from RIM after a protracted legal battle.
NTP’s outside lawyer, Ron Epstein, told AllThingsD that his client was compensated but didn’t offer any details on the amount the company received.
“NTP was happy with the outcome,” said Epstein, who heads Redwood City, Calif.-based Epicenter IP Group. The deal, NTP said, provides broad coverage for all parties to NTP’s patents, including eight related to wireless delivery of email. NTP’s suits, which had been on hold, have now been dismissed.
With the pact — and past deals with Nokia and RIM — NTP has now reached licensing agreements with most of the wireless industry, though there are still a few firms Epstein says the company is approaching about licenses.
What’s unique about the deal, Epstein said, was the fact that NTP managed to get all of the parties to come together and work out a single settlement. That, he said, was a first in his 20 years of practicing patent law.
“We believe this is potentially a new model for how these significant patent portfolios might be licensed,” Epstein said.
A Guide to the Legislative History of the America Invents Act
Guide to History of America Invents Act
Joe Matal, Judiciary Committee Counsel to Senator Jon Kyl, recently published a comprehensive guide to the legislative history of the AIA in the Federal Circuit Bar Journal. Part I addresses the portions of the AIA that relate to applications before a patent issues, while Part II deals with the portions that apply after a patent is granted.
Part I of II
U.S. Senate, 2012; 21 Fed. Cir. B.J. 435 (2012)
Abstract:
The article examines the origins and the legislative commentary on the provisions of the Leahy-Smith America Invents Act, a comprehensive patent-reform law that was enacted on September 16, 2011. The AIA adopts the first-to-file system of patent priority, modifies the defintion of prior art, and creates several new post-issuance administrative proceedings and amends existing proceedings. Part I of the article focuses on the AIA's changes to sections 102 and 103 of title 35 and related issues. The article is organized by the sections of title 35 that are significantly amended by the AIA, and then by the uncodified sections of the AIA.
http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2064740
Part II of II
U.S. Senate, 2012; 21 Fed. Cir. B.J. 539 (2012)
Abstract:
This article is the second in a two-part series examining the origins and the legislative commentary on the provisions of the Leahy-Smith America Invents Act. This second article addresses the AIA's enactment of a prior-user right, its repeal of the false-marking qui tam action and the best-mode defense and the 1952 Act's deceptive-intent restrictions, its authorization of supplemental examination and post-grant review of patents and special review of business-method patents, its revisions to inter partes proceedings, its limits on joinder of defendants and on use of evidence relating to advice of counsel, its authorization of virtual marking of patents, and its modification of the deadline for seeknig a patent-term extension. The article is organized by the sections of title 35 that are significantly amended by the AIA, and then by the uncodified sections of the AIA.
AIA Patent Fee Proposal Released
USPTO Proposed Fee Modifications
The USPTO, on February 7th 2012, released its proposed fee modifications under the changes through the America Invents Act, AIA. Details of the proposal that was submitted to the Patent Public Advisory Committee can be found here:
HR 3889 Proposed Amendments - Design Patent Law
Review of Design Patent Law Changes
A recent note on the PATENTLYO website mentioned the proposed house bill H.R. 3889 (formerly H.R. 3059): (PARTS) Promoting Automotive Repair, Trade, and Sales Act, that would affect design patents term of enforcement. The proposed change would reduce the time period from 14 years to 30 months. The group Keep Auto Parts Affordable supports the bill and provides some rationale here: The Looming Threat
Testimony from the Property Casualty Insurers Association can be found here: Testimony of the Property Casualty Insurers Association of America House Judiciary Committee Hearing “Design Patents and Auto Replacement Parts”
We provide the following review on the testimony with selected text from the testimony and added bulleted comments below them. We feel this topic warrants further discussion as it has potential ramifications beyond the intended purpose. It sets a potentially dangerous president where intellectual property rights are not equally applied for the same idea or invention.
Senate Approves HR 1249
Senate Approves America Invents Act
The U.S. Senate voted to approve H.R. 1249 "The America Invents Act" by a vote of 89-9. The bill will now head to President Obama's desk. He has indicated that he will sign it into law.
The following amendments were voted on by the Senate prior to voting on H.R. 1249 and all were struck down.
HR 1249 USPTO Fee Increases
Patent Fees to Increase under HR 1249
Most of the provisions of the Leahy-Smith Act will take some time to become effective. The change to a first-to-file regime from first-to-invent will only apply to patent applications with an "effective filing date" that is 18-months from enactment or thereafter. Other provisions will take effect almost immediately. A summary of the bill and all it's sections can be found here: section_summary_26jul2011.pdf
The following provisions, as stated in the act will take effect almost immediately:
- Implements 15% surcharge on all patent fees, effective 10 days after enactment
- Establishes prioritized examination fee of $4,800 (above usual fees) with 50% reduction for small entities, effective 10 days after enactment.
- Defines “micro entity” (to include universities) and provides for 75% fee reduction for those applicants.
America Invents Act HR 1249 Final Vote Likely Today
U.S. Senate voting on America Invents Act
The U.S. Senate is currently considering amendments to H.R. 1249, the "America Invents Act of 2011." The vote is scheduled to come up today at 4:00 pm
Final Amendments Proposed:
i) Sen. Coburn has proposed an amendment to absolutely prohibit fee diversion — allowing the USPTO to spend the fees it collects.
ii) H.R. 1249 includes a provision that would retroactively lengthen the deadline for applying for a patent term extension. Sen. Sessions has proposed an amendment stripping that provision from the bill. This particular provision of the reform has no other purpose than to reinstate the The Medicine Company’s (MDCO’s) patent covering its Angiomax drug. The law firm WilmerHale is on the hook for substantial malpractice damages due to the filing debacle. These two firms have spent around $20 million lobbying Congress on this issue.
iii) Sen. Cantwell has proposed separate amendments — The first would eliminate the the business method patent ‘transitional program.’ Failing that, a separate amendment would limit the scope of the program only to “patents claiming abstract business methods” and not to patents covering “technological” or “nonfinancial” inventions.
iv) Sen. Paul has proposed an amendment to include the statement that “It is the sense of Congress that Secretary of the Treasury Timothy Geithner no longer holds the confidence of Congress or of the people of the United States.”
v) Sen. Johnson proposed an amendment to limit all regulatory action by any federal agency until the US unemployment rate drops to 7.7%.
America Invents Act impacts Venture Funding
Venture Capitol Funding impact of America Invents Act
The "America Invents Act" is poised to pass the senate. A review of the effects of one change to the patent system, the new Post Grant Review process can be found here. It has the potential for profound impact on Venture Capitol funding.
HR 1249 Senate Cloture Vote
H.R. 1249 America Invents Act
The U.S. Senate will resume consideration of the motion to proceed to H.R.1249, the America Invents Act. Coverage is on CSPAN-2 scheduled for 10:00 am (Eastern) today when the Senate convenes.
On 9/6/2011 Senate floor actions. Status: Cloture on the motion to proceed to the measure invoked in Senate by Yea-Nay Vote. 93 - 5. Cloture is the procedure by which the Senate can vote to place a time limit on consideration of a bill or other matter, and thereby overcome a filibuster. Under the cloture rule (Rule XXII), the Senate may limit consideration of a pending matter to 30 additional hours, but only by vote of three-fifths of the full Senate, normally 60 votes.
Patent Reform 2011 Vote
America Invents Act
The "America Invents Act" or the Patent Reform Bill of 2011 is scheduled for a vote on Tuesday September 6th. The Senate has agreed to use the House bill H.R. 1249 in leu of it's own bill (S.23). A good summary and comments can be found at the PatentlyO.com blog site:
Patent Reform 2011: Vote Scheduled At the Conclusion of Labor Day
Patent Trolls
Google-Motorola, let the Patent Arms Race Begin!
In a recent article entitled Google-Motorola Deal Highlights Patent Arms Race By Peter Svensson, AP Technology Writer on Manufacturing.Net - August 18, 2011, we find another discussion on how companies such as Google don't think that patents are valid property in and of themselves. The core belief is that companies, and by the same logic, individual inventors or small entities, that don't build the products using the technology for which they own patents should basically have no rights in using that patent property to protect their inventions.
Google buys Motorola Mobility
Google makes bid for Motorola Mobility and it's Patents
Yesterday, 8-15-2011. Google announced that it had agreed to buy Motorola Mobility to, as CEO Larry Page puts it, Supercharge Android. Along with the mobile phones and other technology another key component of the deal is the acquisition of the Motorola Patent portfolio.
Google Patent Reform
Google seems to find competing in the space of ideas difficult
The new patent reform changes currently under consideration need to be clearly understood by small and medium sized entities. Even companies the size of Google with all its resources find competing in the space of ideas difficult. The reason we have patents is to protect the rights of the inventor, big or small. Google has indicated these rights have value through the recent acquisition of more than 1000 patents from IBM. They lost out on Nortel's patent portfolio at auction to a consortium of companies including; Apple, Microsoft, Research in Motion, Ericsson, Sony, and EMC. Why did Google want the Nortel and/or IBM patents? Back in April, 2011, Google indicated in its online blog posted by Kent Walker, Senior Vice President & General Counsel, (Patents & Innovation) that having patents is a good idea, one can protect one's ideas and property and ward off lawsuits.
HR 1249 Ammendment Vote Summary
A summary of the House vote for amendments to HR 1249
Click the READMORE link below to see the full summary
HR 1249 Passes House
The U.S. Congress passes their version of the "America Invents Act"
The U.S. Congress on June 23rd, 2011 passed their version of the "America Invents Act" or H.R. 1249 by a vote of 304-117. The Senate passed their version, S. 23, in a 95-5 vote back in March. The two versions of the bill have to be reconciled before a final bill can be sent to the White House for President Barack Obama’s signature. Read more about the latest bill here: Patent Agency Would Gain Control of Funding Under House Measure
HR 1249 Current Amendments
America Invents Act H.R. 1249 and S.23
While the current status of the "America Invents Act" (H.R. 1249 & S.23) has a postponement of the Rules Hearing amendments are being filed following the amendment rules for H.R. 1249. A list of the current amendments to H.R. 1249 including a summary of each can be found here.
Judiciary Committee Approves Patent Reform Proposal
Manager's amendment now included in H.R. 1249
In continuing coverage of the House Patent Reform Act, H.R. 1249, the Judiciary Committee of the House of Representatives approved the a bill in a 32-3 vote.
Patent Fast Track Examination Begins May 4th - $4000
Track-1 Prioritized Filing System Starts
A new Patent filing prioritization system, known as Track-1, will begin as of May 4th, 2011.
US House introduces Patent Reform Bill
Patent Reform Bill H.R. 1249
On Wednesday, March 30th, 2011 the United States House of Representatives introdueced their version of the "American Invents Act" aka Patent Reform Act of 2011 (click here for copy of HR1249).
More to come on the reactions and differences between the Senate Bill (S.23) and H.R. 1249.
Inventor
In-ven-tor n. a person who invents
As we continue our review of Senate Bill 23 - Patent Reform Act of 2011 - a key definition to keep in mind is that of Inventor. Webster's New Universal Unabridged Dictionary, the 4.5 inch thick paper version no less, offers the following definition: In-ven-tor n. a person who invents, esp. one who devises some new process, appliance, machine, or article; one who makes inventions.
Simple right?
Senate Passes 2011 Patent Reform Act
Inventors need to file early under Patent Reform Act
On March 8th, 2011 the U.S. Senate passed the Patent Reform Act of 2011 (S.23) by a vote of 95 - 5. Now it is on to the House where a similar bill is being crafted. This is an important bill for small businesses, individual inventors, and Venture Capitalists that invest in intellectual property, to keep tabs on and review. (Get the full bill document here)
Patent Reform Act Cloture Vote
Senate votes on Patent Reform Act
A cloture vote on S23, the Patent Reform Act of 2011, is scheduled for Monday, April 7th, some time after 5:30 pm, which could end debate on this bill.
This bill seeks to amend title 35, United States Code, to provide for patent reform. We wanted to bring the bill to attention. One key issue in the bill is the conversion from a First-to-Invent process to a First-to-File. This alone has ramifications that will affect small businesses and individual inventors. In the coming days we will follow this and post more feedback.
More information on this critical bill can be found here: US Senate Bill S23
Invention - America
America needs to Invent!
Thomas Jefferson once wrote to his daughter Martha, in 1787, that because America did not posess Europe’s vast resources, we are obliged to invent and execute, to find means within ourselves, and not to lean on others.
Building a BIGGER Box
The importance of Continued Learning and Growth
Specialized = Limited? Or is it depth? It is key to have depth if one specializes and in the end we ALL are limited. However, there is nothing stopping you from developing multiple areas of "specialization." Multiply the dimensions of YOUR box through time and effort. The result will be both depth and breadth. In the "olden days" there used to be a well established apprentice program for many type of jobs. One might know how to build a fire, heat a piece of metal, and swing a hammer, but knowing how to and having the skills to make that piece of metal into a fine sword takes more. Today's society is focused on "speed" in all kinds of ways that limit the ability of people to grow properly. The internet, technology like cell phones and computers, can cause distractions and overload that minimize the depth to which some have knowledge. In the end these are all tools and what is most important is the person using those tools. Like the apprentices of old knowledge must be gained over time and through training, learning from mentors and experts, and simply being apprentices. Remember to never stop learning!
Further Reading on the Topic:
Why You Should (probably) Hire Specialists The Generalist Bias3D Scan to Print Web App
A company called Volumental out of Stockholm Sweden is developing a browser-based app that will use a Depth Camera to help you grab 3D representations of real objects, in full color right at your desk. Depth Cameras are cameras that have two lenses like those for Kinect. The process involves only the camera, your browser and an online printing service. The app connects to the scanner to take the measurements and then make models through the online service.

Volumental toutes the technology on their KickStarter page as "the world's first that allows users to create usable, shareable 3D models of your living room sofa, grandfather, or a kitchen appliance all without leaving your browser window." While these object might be "fun" to do there is a more serious purpose this technology may be put to, Reverse Engineering. The ability to convert existing objects into a 3D scan and subsequently into a water-tight and closed, printable model would allow both the printing of the object for physical needs but may also allow for digital representations of the objects to be utilized for Virtual Prototyping via simulations.
The technology in development and still in need of funding but there is much promise for its future use. More details on their KickStarter page here:
http://www.kickstarter.com/projects/volumental/the-3d-scan-to-print-web-app
Googles Adidas Shoes that Talk
Introducing...The Shoogle!

Google and Adidas have partnered for the development of Shoes that Talk. Google turned them into an actual "walking-and-talking shoe." I can see talking but, do they really walk for you? How lazy are we getting?
Percifield says, "We developed a shoe that could talk and tell you things--that could pick up enough information about your exercise, whether you're walking, running, moving fast or slow." If you aren't aware that your walking, running, moving "fast or slow", until your shoe points it out, then I think you may want to go lie down.
The team put a speaker on the tongue of the shoe to "give feedback and motivation as you move throughout your day." Really? We need motivating "wisdom" from a pair of sneakers? And, it will point out your activity, or lack thereof, to your social network! "It has your own social network feed so all of your friends can see how well you treat your shoes--and what your shoe says about you." Great! Now your shoes can call you out via Twitter or Facebook. Why not add a webcam so live video feeds of, well your "feeds", can be shared as well? "Oh, look at him now, pigging out on cheese doodles and red bull!" Percifield says "We're doing real-time, right-in-your-face feedback." Ain't technology wonderful?
And what does the future hold for the Shoogle? Well, how about replacing your personality through "an inanimate object (having) a personality of its own?" Personally, I think I'll hold out for the boxer shorts. Maybe they could tell you something useful. They will give hope to millions of women seeking a direct answer to the age old question..."does this outfit make me look fat?"
Link to Article: Here
Knowvention Plastics in Medical Devices PIMD 2012
Plastics in Medical Devices 2012 Conference
Come visit us at the Plastics in Medical Devices 2012 Conference. June 11th through the 13th in Cleveland. Kevin Harper, President of Knowvention, will be presenting and participating on a discussion Panel regarding the Front End of Innovation. For anyone interested there is more information provided in the following link:
BioOhio Expo
BioOhio Suppliers and Service Providers Online Expo.
Knowvention is proud to be a Sponsor for the BioOhio Suppliers and Service Providers Online Expo. Ohio is home to more than 1,250 bioscience-related organizations, from start-ups and emerging stars to fabled research institutions and Fortune 500 mainstays. BioOhio helps you connect with our state's expanding bioscience community. You can sign up for the event that runs now through April. Just follow this link: http://www.jujama.com/bioohiosignup.aspx
To learn more about BioOhio go to: http://www.bioohio.com
TechKnow Introduction
Welcome to TechKnow!
This is Knowvention's news section developed to share information on a wide variety of topics of interest. Topics include: Innovation, invention, business, marketing, product development, engineering, technology, computers, software, ideas, patents, and more!
Stay tuned!
